|
|
|
|
 |
|
| Welcome to the Clinical Testing Solutions newsletter, February 2022. In this edition you'll find a blog post and podcast on SARS‑CoV‑2 Omicron variant. Read our newly published blog posts on COVID‑19 testing during flu season and discover interesting information about our next generation PCR‑based COVID‑19 test kits. Learn more on the In Vitro Diagnostic Regulation (IVDR) and how Thermo Fisher Scientific is responding the new legislation, including its impact on molecular pathology laboratories. |
|
|
|
|
|
|
|
|
Omicron: Why scientists are concerned
The latest SARS‑CoV‑2 Variant of Concern has taken the headlines by storm. Here's why it has scientists and public health officials worried.
Find out more »
|
|
|
|
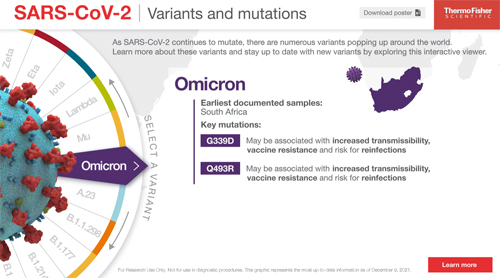
Stay up-to-date with all SARS‑CoV‑2 variants and mutations
Use our interactive viewer to learn more about the latest variants and mutations popping up around the world.
Access our interactive viewer »
|
|
|
|
|
BBC radio podcast: The Omicron variant, testing solutions, and the pandemic response
Featuring Dr. Manoj Ghandi, Director of Medical and Scientific Affairs, Thermo Fisher Scientific. |
|
|
| COVID-19 testing during flu season |
|

Reducing testing stress: The benefits of multiplex testing during flu season
Dr. Anami Patel, Poplar Healthcare, shared his lab's journey adopting multiplex technology to determine whether patients are infected with COVID-19 or flu with a single test.
Read blog » |
|

When COVID-19 meets flu, reliable multiplex detection methods are critical
Manoj Gandhi, Thermo Fisher Scientific, talks about the similarities and differences between COVID-19 and flu, highlighting the importance of testing in controlling viral transmission and staying ahead of demand.
Read blog » |
|
|
|
|
Diverse testing and surveillance solutions in the quest to control COVID-19
The ability to detect, examine, and better understand SARS-CoV-2 behaviour within communities is critical to gaining control of the pandemic.
Read blog »
|
|
|
|
How COVID-19 tests are staying ahead of SARS-CoV-2 variants
As COVID-19 continues to evolve, testing capabilities must stay ahead of the changing virus.
Read blog »
|
|
|
| The next generation of COVID-19 testing |
|
 |
|
Next generation COVID-19 testing
The Applied Biosystems™ TaqPath™ COVID-19 2.0 Kits help laboratories quickly and accurately diagnose COVID-19. Learn more about our gold standard testing solutions.
Read blog » |
|
Say hello to the TaqPath™ COVID-19 RNase P Combo Kit 2.0
Multiplexed, highly sensitive, RT-PCR assay for the qualitative detection of
SARS-CoV-2 RNA
Read blog » |
|
|
Say hello to the TaqPath™ COVID-19 Fast PCR Combo Kit 2.0
Fast RT‐PCR assay for the qualitative detection and characterisation of SARS-CoV-2 in saliva
Read blog » |
|
|
|
From raw saliva to interpreted results in approximately two hours
Explore the workflow of the Applied Biosystems™ TaqPath™ COVID-19 Fast PCR 2.0 Combo Kit, a direct-to-PCR COVID-19 test. See how you can go from sample to results (bypassing RNA extraction) in approximately 2 hours using your current Applied Biosystems™ QuantStudio™ real-time PCR instrumentation.
Watch video »
|
|
|
|
|
On-demand talks from ASLM2021: Evolution of viral pathogens
During our ASLM2021 symposiums, customers shared their experiences and latest approaches in testing technology and surveillance options for understanding the evolution of viral pathogens, including SARS‑CoV‑2 and HIV.
Watch webinar »
|
|
|
|
ASLM round-up: As COVID-19 cases in Africa surge, testing and surveillance remain critical
Learn more about the content of the scientific discussion that took place during our symposiums at ASLM2021, with another opportunity to watch the on-demand talks.
Read blog »
|
|
|
|
Webinar: How molecular labs can handle the new IVDR regulation
In this virtual event, we discussed the value of the new In Vitro Diagnostic Regulation (IVDR) legislation and the impact for molecular pathology laboratories.
Watch webinar »
|
|
|
|
|
|
|